Background: Activation of p53 is one of major pathways by which DNA damaging agents (DDA) such as radiation and chemotherapy cause toxicity in normal tissues and it induces a cascade of events that eventually leads to cell senescence or cell death. We have reported that a brief pretreatment with low dose arsenic (LDA), by temporarily and reversibly downregulating p53 at the time of treatment with DDA, reduces the normal tissue toxicity without compromising tumor response to treatment. This protective effect is selective to normal tissues, as it requires functional p53. Though not every cancer cell has detectable p53 mutations, essentially every cancer cell has dysfunctional p53. Therefore most cancer cells will not be protected by this strategy. Genomic instability and inability to repair DNA damage from DDA in the hematopoietic stem cells have been attributed to the development of therapy-induced myelodysplastic syndrome (tMDS) and acute myeloid leukemia (AML). We have also been studying the effect of LDA on the genome in the setting of cancer therapy. We have reported that LDA pretreatment significantly reduces radiation-induced DNA double strand breaks (DSBs) and apoptosis in normal cells both in-vitro and in-vivo. Persistent DNA damage such as DSBs can trigger genomic instability and can be prevented by proper DNA repair. Our previous work using comet assay to quantify DNA damage after radiation has indicated that DNA repair capacity is enhanced by LDA pretreatment. A role for LDA in maintaining genomic integrity has been implicated in our in-vitro studies, where we found that LDA protected telomeres from enhanced erosion by DDA in Concanavalin A-activated normal human lymphocytes, and that LDA reduced spontaneous and radiation-induced mutations in mouse embryonic stem cells. Yet, whether this p53 downregulation-based strategy helps genome maintenance during cancer treatment using DDA has not been investigated in-vivo. CBA/Ca mice have 15-25% incidence of AML after 3 Gy of total body ionizing radiation (IR). About 95% of mice that develop radiation-induced AML (rAML) have a deletion on chromosome 2 encompassing the PU.1 gene. Since PU.1 deletion is a critical contributor to and a useful surrogate marker for leukemogenesis in the murine rAML model, we tested a hypothesis whether pretreatment with LDA before IR helps maintain genomic integrity by evaluating bone marrow cells for PU.1 gene deletion.
Method: One hundred twenty mice were randomized into four groups: PBS+sham IR (control), LDA+sham IR, PBS+IR and LDA+IR. Prior to sham or 3 Gy of IR, CBA/Ca mice were injected with either PBS or LDA intraperitoneally at the dose of 0.4mg/kg for 3 days. At 7, 30 and 180 days after radiation, bone marrow cells were collected from femurs and fixed with Carnoy's Fixative. To assess the effect of LDA on PU.1 gene deletion, fluorescence in-situ hybridization (FISH) assay was performed. An ATTO550 labeled PU.1 probe was designed and used to detect deletions that occur in 2qE1 and involve the PU.1 gene locus, as well as two 6-FAM labeled probes for centromere and telomere respectively. Four to five hundred cells were analyzed for each mouse. Statistical significance was determined from a two-way analysis of variance in log units using SAS Version 9.4.
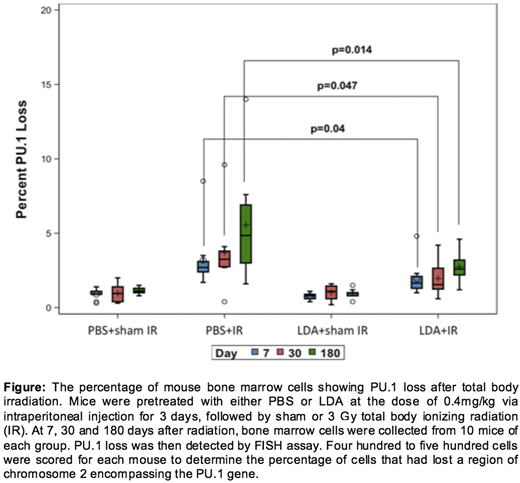
Result: We successfully established the FISH assay that can specifically detect the PU.1 gene not only in metaphase cells but also in interphase cells. As shown in the figure, mice in the LDA+IR group have significantly fewer bone marrow cells exhibiting PU.1 gene deletion compared with PBS+IR group at all three time points examined (Day 7: 2±1.2% vs 3.7±2.6%, P=0.047; Day 30: 1.9±1.1% vs 3.2±1.9%, P=0.040; Day 180: 2.8±1.0% vs 5.6±3.5%, P=0.014). LDA treatment alone has a negligible effect on PU.1 loss as compared to the control group.
Conclusion: Our result suggests that LDA pretreatment protects genomic integrity following IR treatment in-vivo. As the development of rAML is a multi-step process, the impact of LDA pretreatment on the actual incidence of secondary malignancy needs further validation in animal models. The genome-protective effect of LDA that we have revealed supports its potential use as a strategy to reduce the development of radiation-induced secondary malignances such as MDS and AML.
Ha:Protectum Oncology: Current Employment, Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal